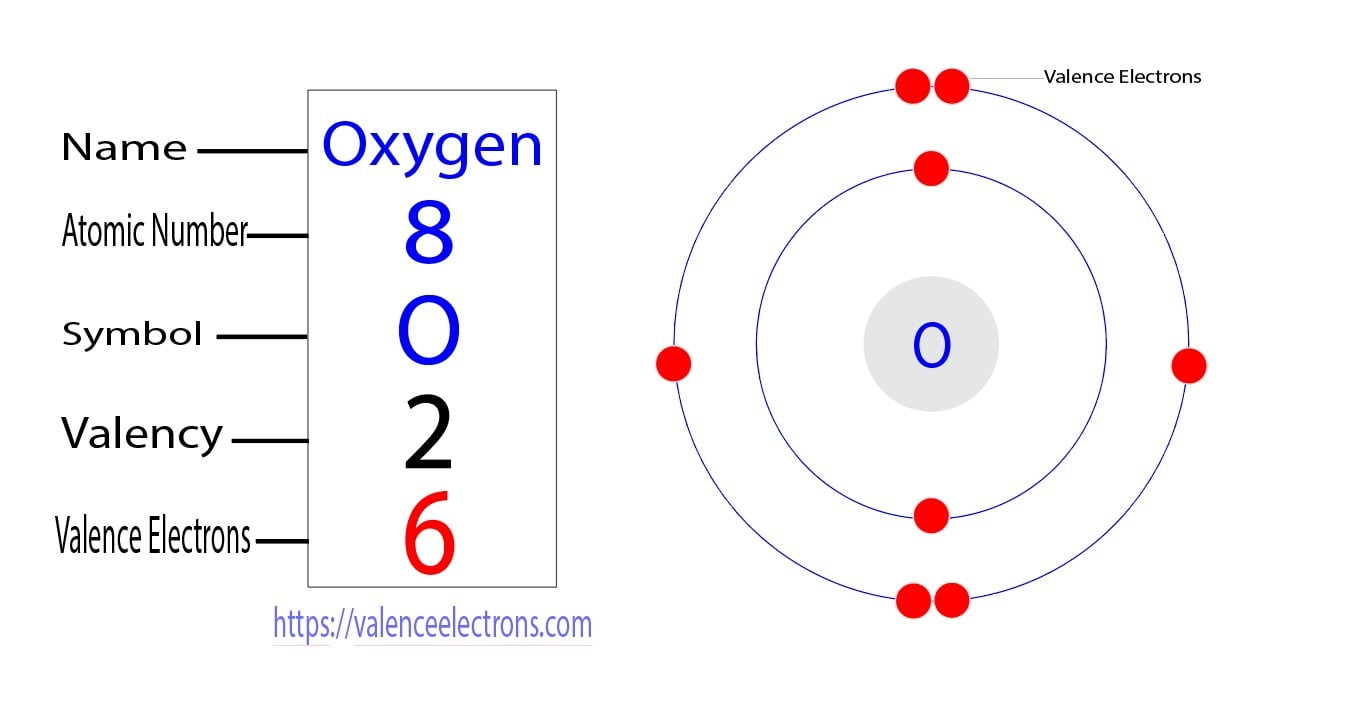
What Is the Oxygen Electron Configuration(O)?
The electron configuration of oxygen is 1s 2 2s 2 2p 4. In O 2, therefore, we need to accommodate twelve valence electrons (six from each oxygen atom) in molecular orbitals. As you can see from the diagram, this places two electrons in antibonding orbitals. Each of these electrons occupies a separate π* orbital because this leads to less.
Bohr Model Drawing Of Oxygen at GetDrawings Free download
Electronic configuration of elements ( Data page-Wikipedia) Electronic configuration for super heavy elements ( Source) Author Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full
Oxygen Electron Configuration (O) with Orbital Diagram
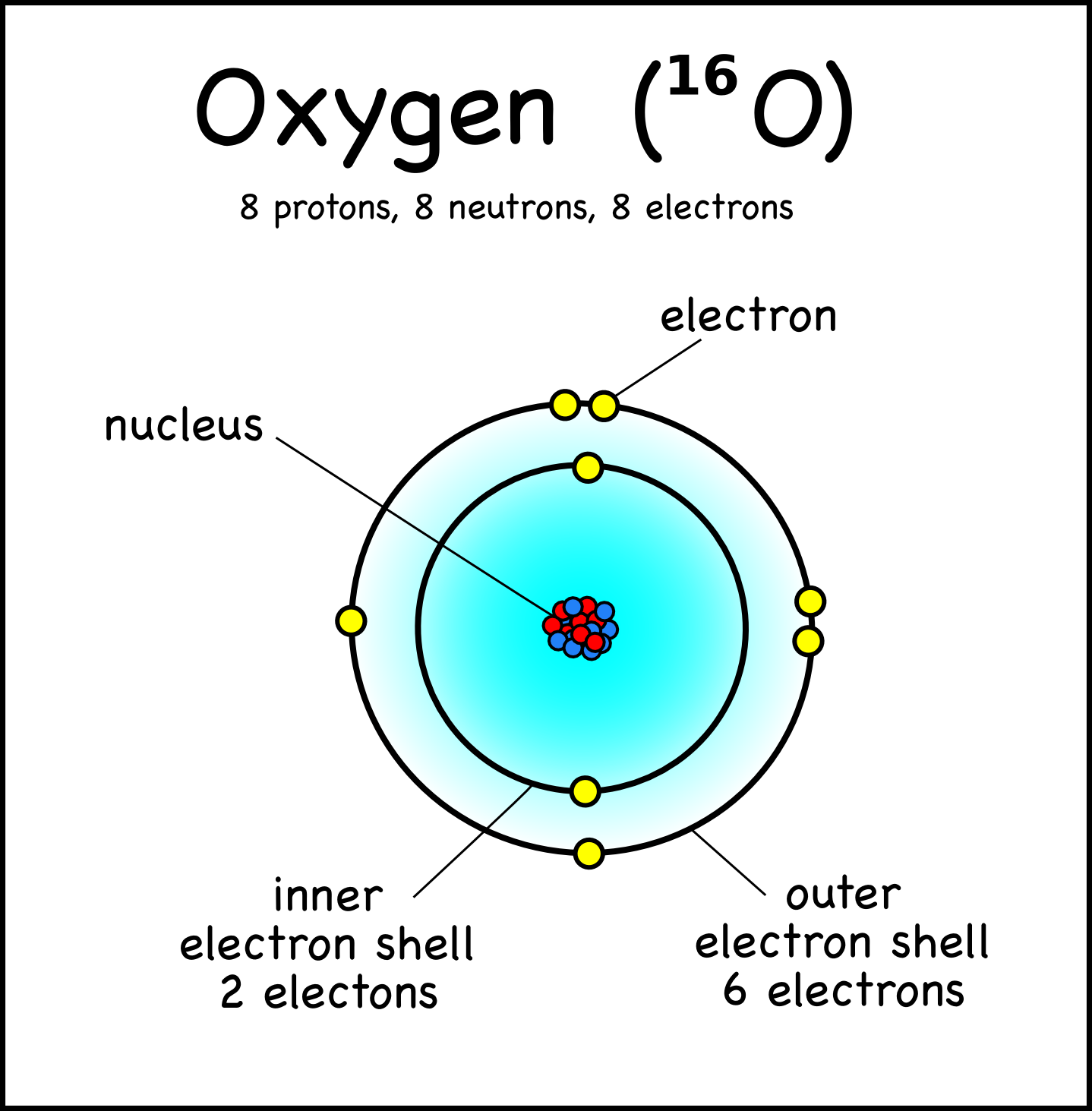
Let's find the electron configuration of Oxygen! A single oxygen atom has 8 protons and 8 electrons, but how do we know where Oxygen puts its electrons, in w.
How to Write Ground State Electron Configuration in Chemistry
Solution Oxygen: Oxygen is an element having an atomic number 8 and an atomic symbol O. It belongs to Group- 16 and second period. It is a highly reactive non-metal. Electronic configuration: The arrangement of electrons into the orbitals of an atom using some fundamental principle is called its electronic configuration.

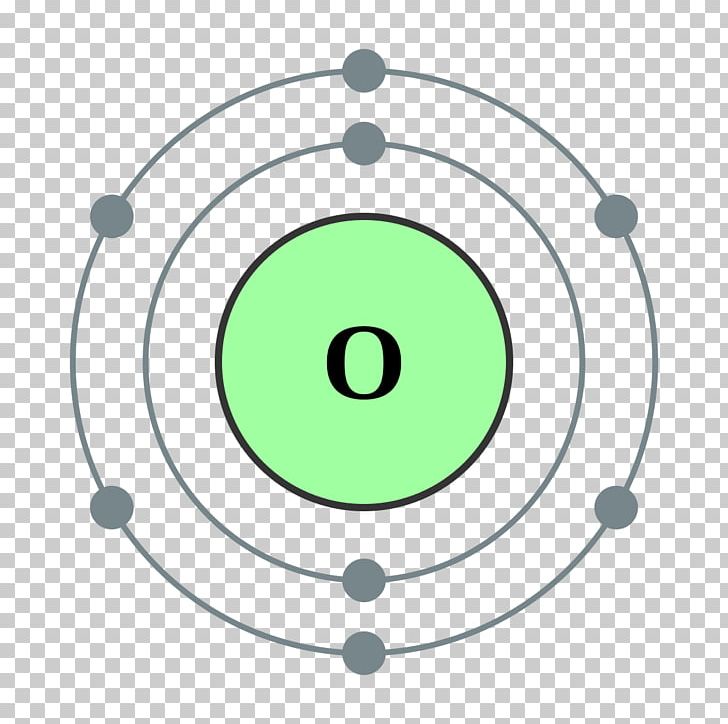
FileElectron shell 008 Oxygen.svg Wikimedia Commons Atom diagram, Electron configuration
Electron Configurations. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating with the loss of or gain.

Diagram representation of the element oxygen Vector Image
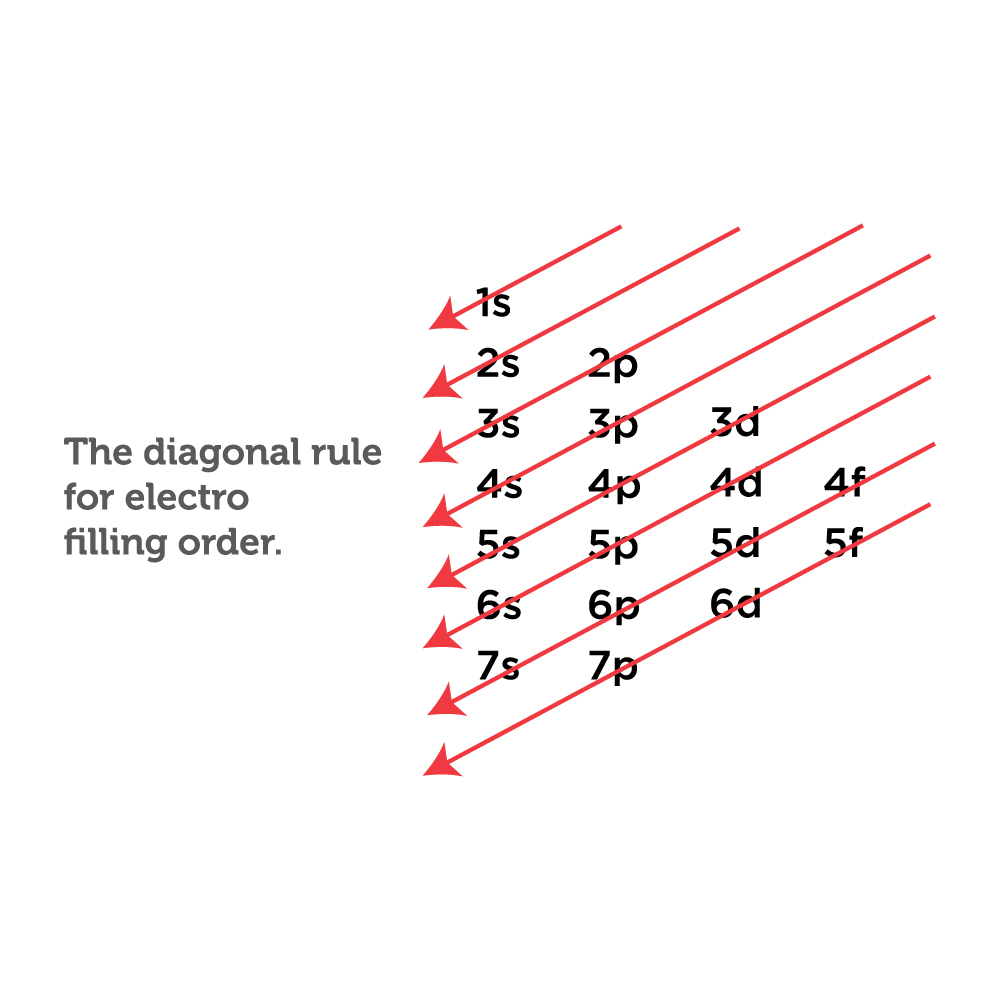
The easiest way to create electron configurations is using an electron configuration table, which is a way of writing down the various orbitals available to electrons. This table is easy to remember, and it makes it possible to generate the electron configuration table for any given element. It looks something like this.
Electron Configuration for Oxygen (O, and O2 ion)
In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83). The periodic table gives the following electron configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p65s2 4d10 5p6 6s2 4f14 5d10 6p3.

Electronic configuration of the oxygen atom Download Scientific Diagram
Oxygen Electron Configuration Wayne Breslyn 724K subscribers Join Subscribe Subscribed 683 Share 130K views 10 years ago A step-by-step description of how to write the electron configuration.
Bohr Model Chemical Element Oxygen Atomic Theory PNG, Clipart, Angle, Area, Atom, Atomic Number
The electron configuration of an element describes how electrons are distributed in its atomic orbitals. Electron configurations of atoms follow a standard notation in which all electron-containing atomic subshells (with the number of electrons they hold written in superscript) are placed in a sequence.

Electron configuration of oxygen ion Lousiana
We expect the two electrons that occupy these two degenerate orbitals to be unpaired, and this molecular electronic configuration for O 2 is in accord with the fact that the oxygen molecule has two unpaired electrons ( Figure \(\PageIndex{10}\)). The presence of two unpaired electrons has proved to be difficult to explain using Lewis structures, but the molecular orbital theory explains it.

What is the Electron Configuration of Oxygen Archives Dynamic Periodic Table of Elements and
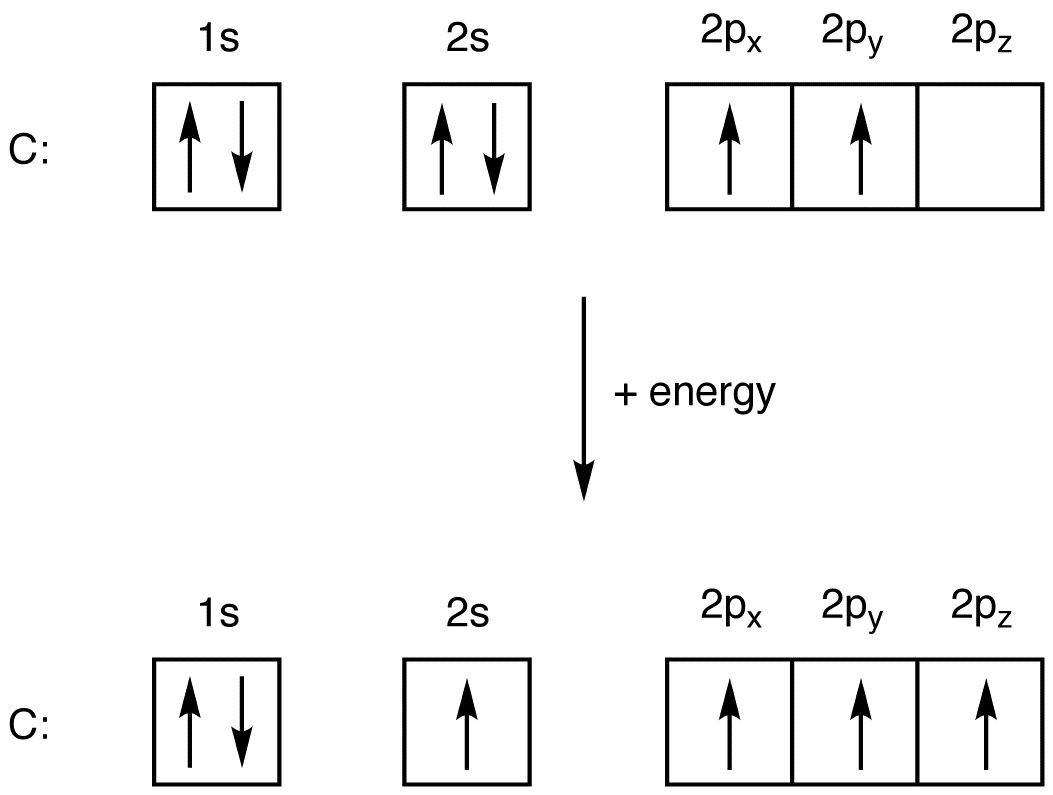
Example 1.6. 3: Carbon and Oxygen. Consider the electron configuration for carbon atoms: 1s 2 2s 2 2p 2: The two 2s electrons will occupy the same orbital, whereas the two 2p electrons will be in different orbital (and aligned the same direction) in accordance with Hund's rule. Consider also the electron configuration of oxygen.

Symbol and electron diagram for Oxygen Royalty Free Vector
The electronic configurations of atoms close atom The smallest part of an element that can exist. help explain the properties of elements and the structure of the periodic table.

Oxygen Bohr Model (Diagram, Steps To Draw) Techiescientist
In this video we will write the electron configuration for O 2-, the Oxide ion. We'll also look at why Oxygen forms a 2- ion and how the electron configurati.
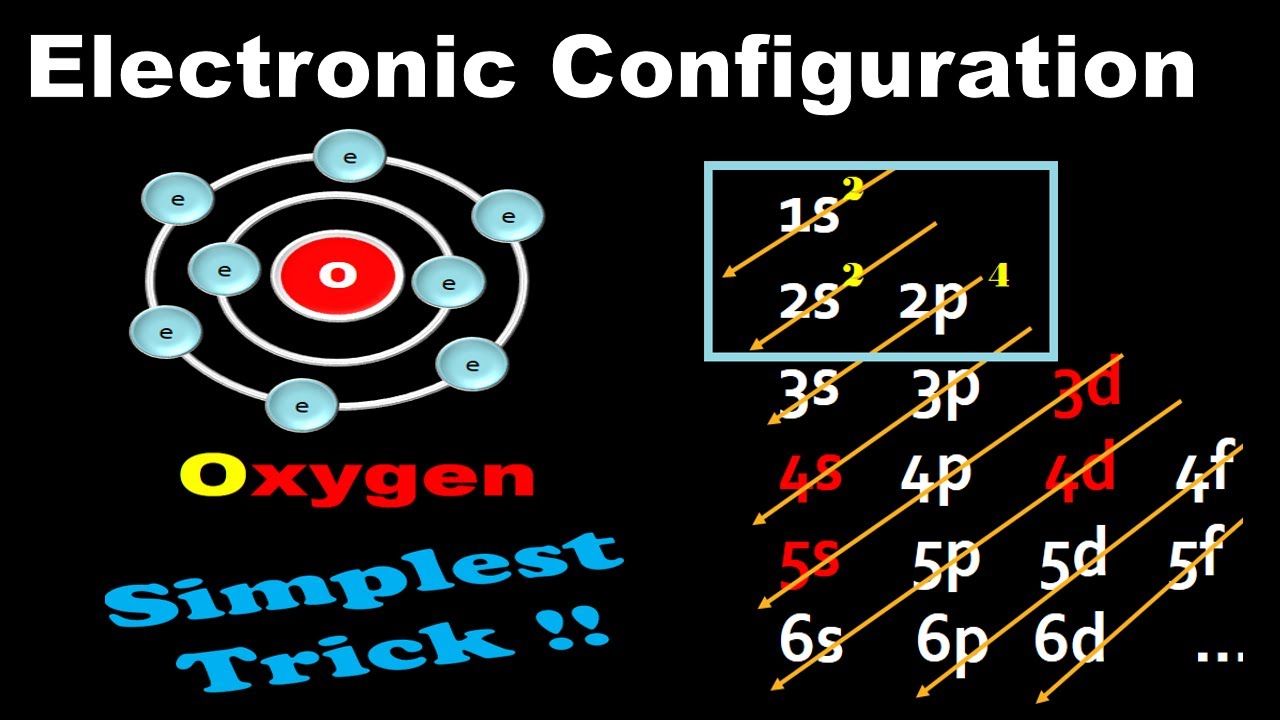
Electronic Configuration For Oxygen spdf Trick Chemistry Atomic Number 8 YouTube
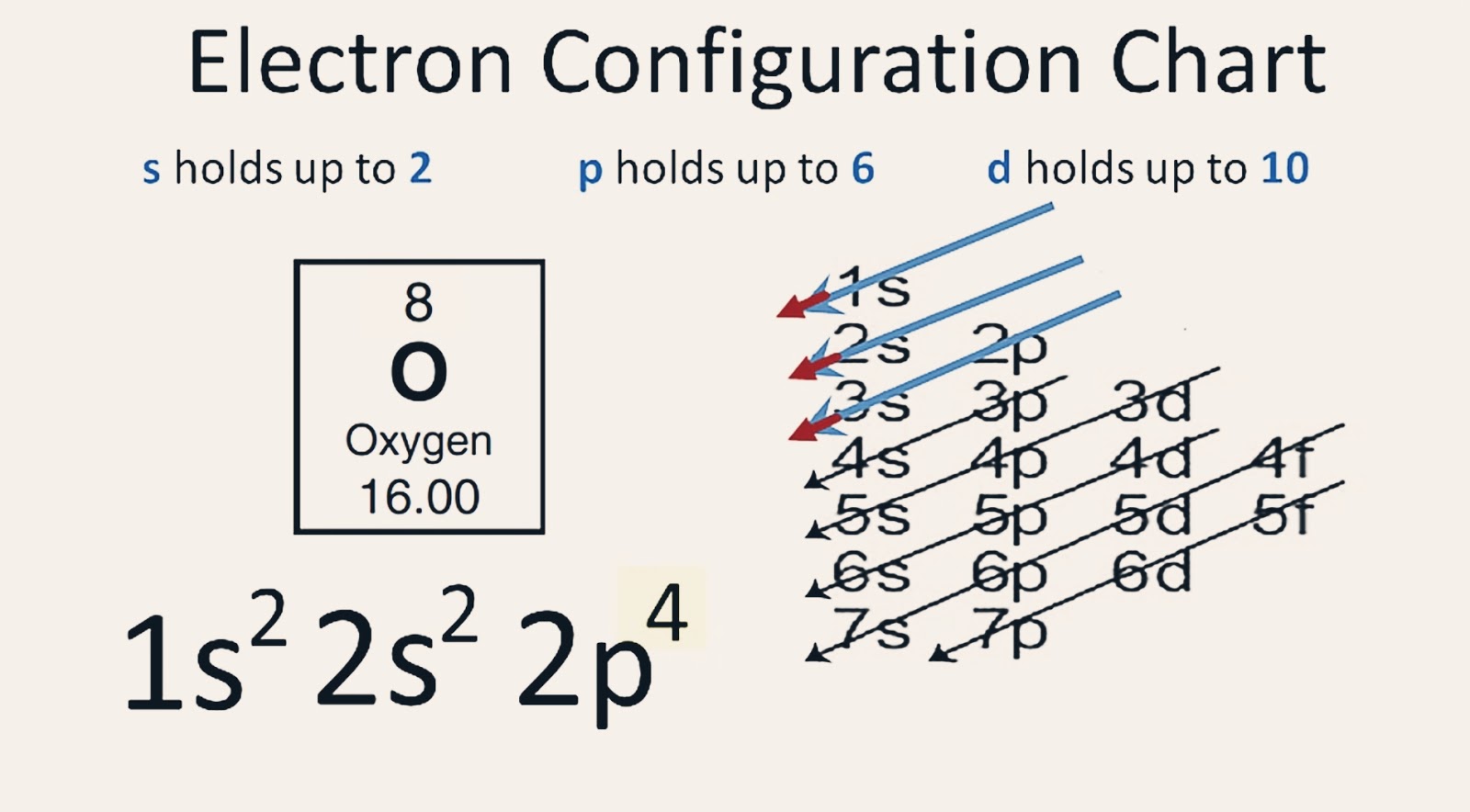
To find the electron configuration of oxygen: Look at the periodic table and find an atomic number of oxygen, which is 8. Fill these 8 electrons in the following order: 1s, 2s, and then 2p. Write the complete electron configuration of oxygen: 1s²2s²2p⁴. Identify the noble gas before oxygen, helium, and write using shorthand notation: [He.

【5 Steps】Oxygen Electron Configuration in Just 5 Steps Electron Configuration of Oxygen(O)
Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom. - helps chemist understanding how elements form chemical bonds. - can be written using the period table or an electron configuration chart. How to Write the Electron Configuration for Oxygen Oxygen is the eighth element with a total of 8 electrons.

Electron Configuration for Oxygen (O, O2 ion)
An electronic configuration is the way in which electrons are arranged in an atom . Electrons in shells Different shells can hold different maximum numbers of electrons. Electrons.