
explain sp3d2 hybridization with an example Brainly.in
Steps to Find Hybridization The chemical process involves the reaction between two or more than two atoms. In chemistry, hybridization is the idea of combining two atomic orbitals to create a brand-new category of hybridized orbitals. Typically, this mixing creates hybrid orbitals with completely distinct energies, morphologies, etc. Hybridization
Electron s{{p}^{3}}{{d}^{2}} hybridization with suitable example.
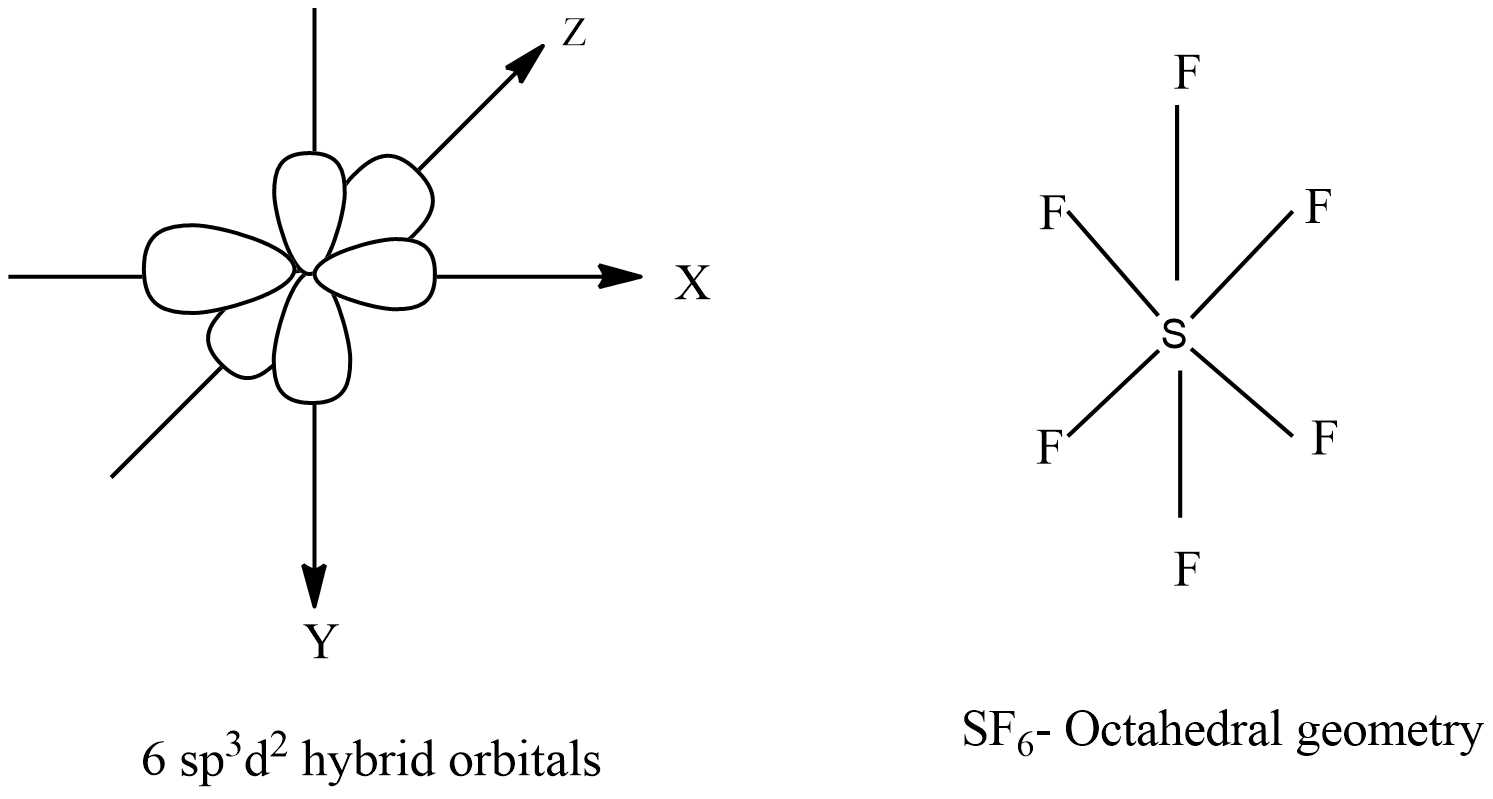
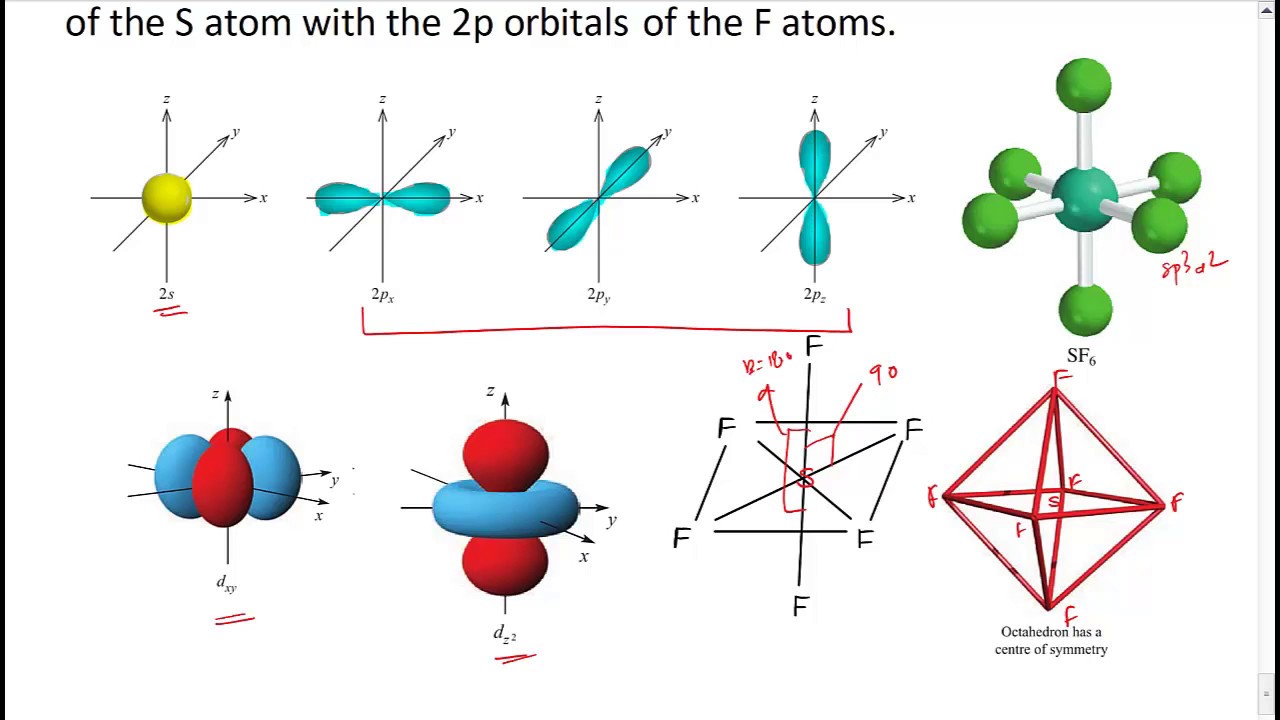
There are no lone pairs of electrons on the central atom. To bond six fluorine atoms, the 3 s orbital, the three 3 p orbitals, and two of the 3 d orbitals form six equivalent sp3d2 hybrid orbitals, each directed toward a different corner of an octahedron. Other atoms that exhibit sp3d2 hybridization include the phosphorus atom in PCl6−,PCl6−,

Hybridization Definition, Types, Rules, Examples
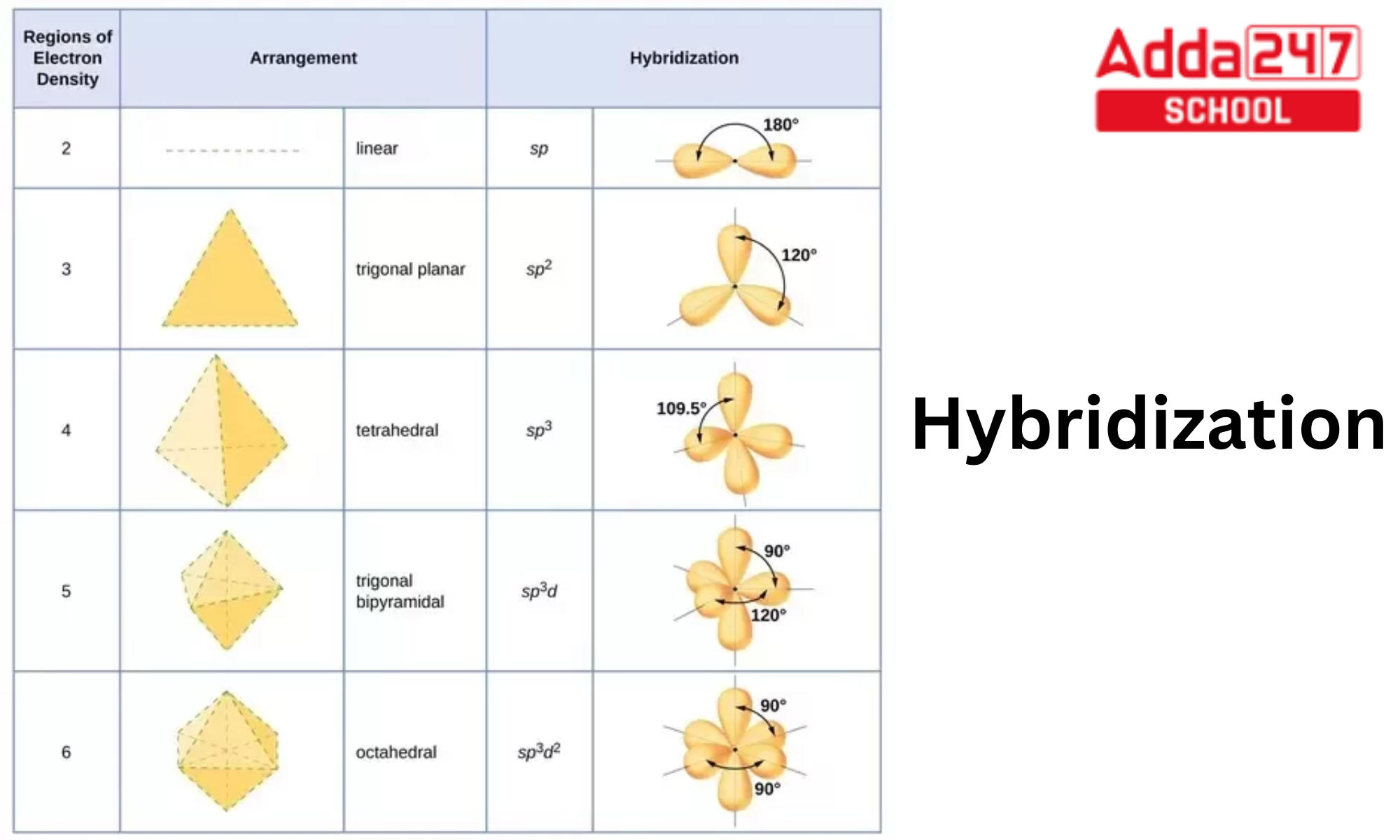
Intermixing of one 's', three 'p' and two 'd' orbitals of almost same energy by giving six identical and degenerate hybrid orbitals is called sp 3 d 2 hybridization. These six sp 3 d 2 orbitals are arranged in octahedral symmetry by making 90° angles to each other.
sp3d and sp3d2 hybriisation chemical bonding chemistry Neeraj dubey YouTube
In this lecture discuss about the Sp3d2 hybridization will all examples. Sp3d2 Hybridization sigma and pi bond. Sp3d2 hybridization in SF6, XeF4, IF5 Gemotry.

Sp3D2 Ujian
sp 3 d2 Hybridization FAQs What Is Hybridization? Redistribution of the energy of orbitals of individual atoms to give orbitals of equivalent energy happens when two atomic orbitals combine to form a hybrid orbital in a molecule. This process is called hybridization .

Hybridization Definition, Types, Rules, Examples
Valence bond theory: Introduction; Hybridization; Types of hybridization; sp, sp 2, sp 3, sp 3 d, sp 3 d 2, sp 3 d 3; VALENCE BOND THEORY (VBT) & HYBRIDIZATION. The valence bond theory was proposed by Heitler and London to explain the formation of covalent bond quantitatively using quantum mechanics. Later on, Linus Pauling improved this theory by introducing the concept of hybridization.

XeF4Xenon tetrafluoridesp3d2 hybridizationStructureShapeBond angleLone pairsAdiChemistry
Examples of Hybridization Things to Remember Previous Year Questions Sample Questions Key Terms: Hybridization, sp, sp 2, sp 3 Hybridization, Atomic Orbitals, Valence Bond Theory, Bond Angle, Molecular Orbital Theory What is Hybridization? [Click Here for Sample Questions]

PPT Molecular Geometry and Polarity Part I Molecular Geometry Valence Bond Theory (B
The sulfur atom in sulfur hexafluoride, SF 6, exhibits sp3d 2 hybridization. A molecule of sulfur hexafluoride has six bonding pairs of electrons connecting six fluorine atoms to a single sulfur atom. There are no lone pairs of electrons on the central atom. To bond six fluorine atoms, the 3 s orbital, the three 3 p orbitals, and two of the 3 d.
What is Hybridization? sp3, sp2, Examples and Formula
sp Hybridization. The beryllium atom in a gaseous BeCl 2 molecule is an example of a central atom with no lone pairs of electrons in a linear arrangement of three atoms. There are two regions of valence electron density in the BeCl 2 molecule that correspond to the two covalent Be-Cl bonds. To accommodate these two electron domains, two of the Be atom's four valence orbitals will mix to.

Sp3d2 Hybridization with all examples/Chemistry with M.Faisal YouTube
sp3d2 hybridization And Examples.What is sp3d2 to hybridization?What is the geometry of sp3d2 to?How many hybrid orbitals are in sp3d2?Sp3d3 hybridizationSp3.

PPT Lecture 11 Covalent Bonding Pt 3 Hybridization (Ch. 9.59.13) PowerPoint Presentation
Hybridization of s and p Orbitals. In BeH 2, we can generate two equivalent orbitals by combining the 2s orbital of beryllium and any one of the three degenerate 2p orbitals. By taking the sum and the difference of Be 2s and 2p z atomic orbitals, for example, we produce two new orbitals with major and minor lobes oriented along the z-axes, as shown in Figure \(\PageIndex{0}\).

Sp3d Orbitals
About Transcript In sp³ hybridization, one s orbital and three p orbitals hybridize to form four sp³ orbitals, each consisting of 25% s character and 75% p character. This type of hybridization is required whenever an atom is surrounded by four groups of electrons. Created by Jay. Questions Tips & Thanks Sort by: Top Voted The Dreamer 9 years ago

Hybridization sp3d2 examples YouTube
Instead of three p orbitals and one s orbital, there are four sp 3 hybrid orbitals. Hybrid orbitals are extremely useful for explaining the characteristics of bonds, as well as predicting the geometry of different molecules. The latter makes use of VSEPR theory (Valence Shell Electron Pair Repulsion).
Sp2 Hybridization Shape
sp hybridization examples (Beryllium chloride, BeCl 2; Acetylene, C 2 H 2) sp 2 (Boron trichoride, BCl 3; Ethylene, C 2 H 4) sp 3 (Methane, CH 4; Ethane, C 2 H 6) sp 3 d (phosphorus pentachloride, PCl 5) sp 3 d 2 (sulfur hexafluoride, SF 6) sp 3 d 3 (Iodine heptafluoride, IF 7)

sp2 sp3 sp3d sp3d2 sp3d3 hybridization examples YouTube
Introduction. The term "sp 3 hybridization" refers to the mixing character of one 2s-orbital and three 2p-orbitals to create four hybrid orbitals with similar characteristics. In order for an atom to be sp 3 hybridized, it must have an s orbital and three p orbitals.. From wave function to the visual representation: Four equivalent sp3 hybrid orbitals, resulting from the combination of one.
What is sphybridization? sphybridization kya hay? JEE/NEET/ IITJAM/ BSC Nursing
July 30, 2019 sp 3 d Hybridization sp 3 d hybridization is shown in phosphorus penta chloride (PCl 5 ). The grounds state and the excited state outer electronic configurations of phosphorus (Z=15) are represented below.